18 e-cigarette products sold and distributed by vapour.com has officially been ‘declared’ by the MHRA – which means all the products have been medically certified and approved by the MHRA (Medicines and Healthcare Products Regulatory Agency).
The MHRA website has listed authorisation of e-cigarettes as under the TPD (Tobacco Products Directive), and is the ‘responsibility of the producer to ensure that their products comply with the TPD requirements. We are currently checking notifications submitted during the transition period for completeness and verifying TPD compliance with producers. Where this review has been completed, the TPD compliance status of products is recorded as ‘declared’ to indicate that the notification is complete and the product has been declared compliant by the producer’.
The MHRA is the official governmental body responsible for regulating NCP’s which are nicotine-containing products, that are now classed as medicinal products. This category includes e-cigarettes which have been found to be one of the most used and successful cessation method for smokers to ditch the nasty habit. Electronic cigarettes have therefore seen a lot of regulation and policy come into effect; in particular since May, when the TPD was officially instigated. Now that e-cigarettes are deemed ‘medicinal’, their purpose is to encourage and actively support companies to submit medicines authorisation applications to ensure they can continue to sell.
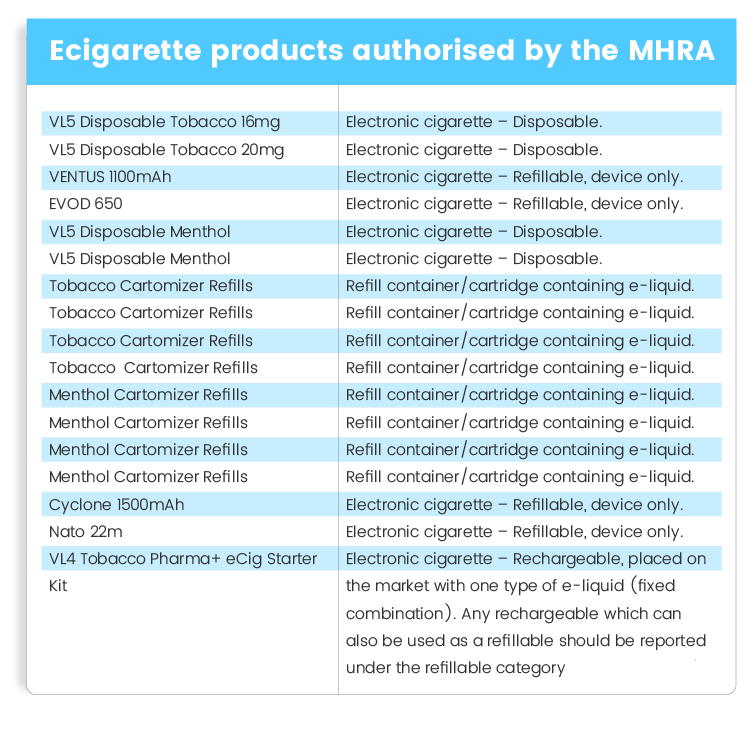
Vapour has had 18 products authorised by the MHRA and they include the following:
Feature image credit: vchal/Shutterstock